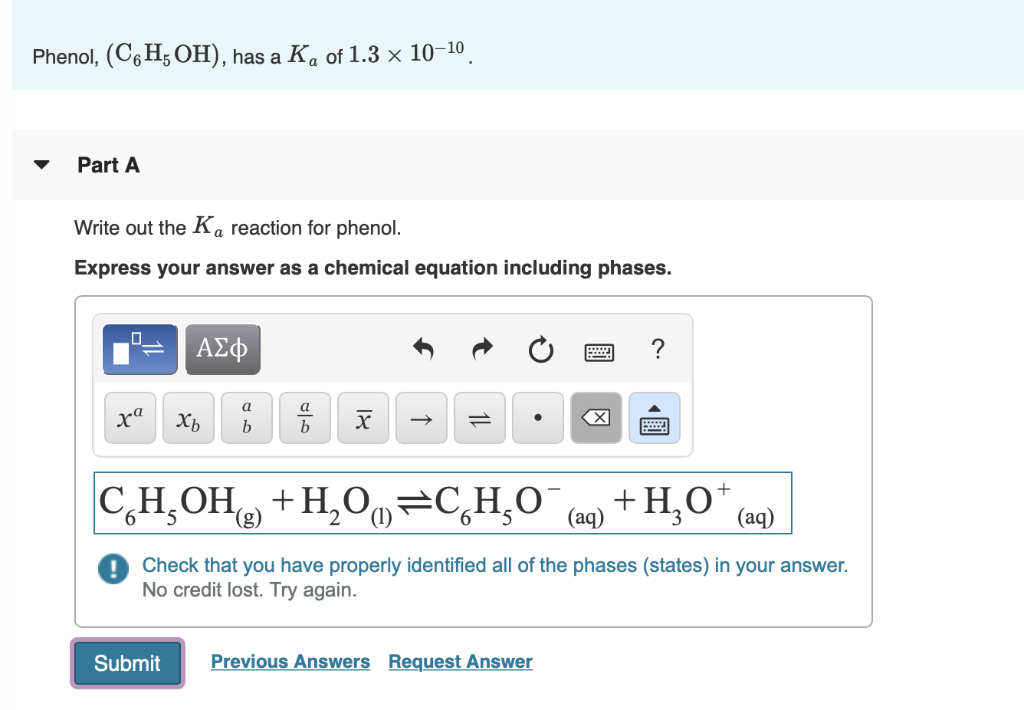
Write Out the Ka Reaction for Phenol.
Determine the Ka of 34-dinitrophenol. See the answer Phenol C6H5OH has a Ka of 131010.
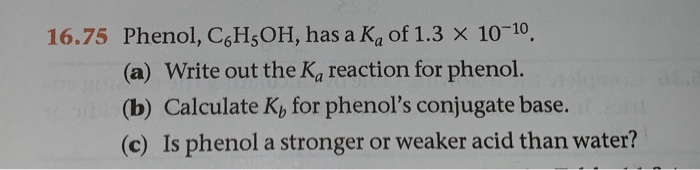
Solved 16 75 Phenol C H5oh Has A Ka Of 1 3 X 10 10 A Chegg Com
Solution for The value of Ka for phenol a weak acid is 100x10-10.
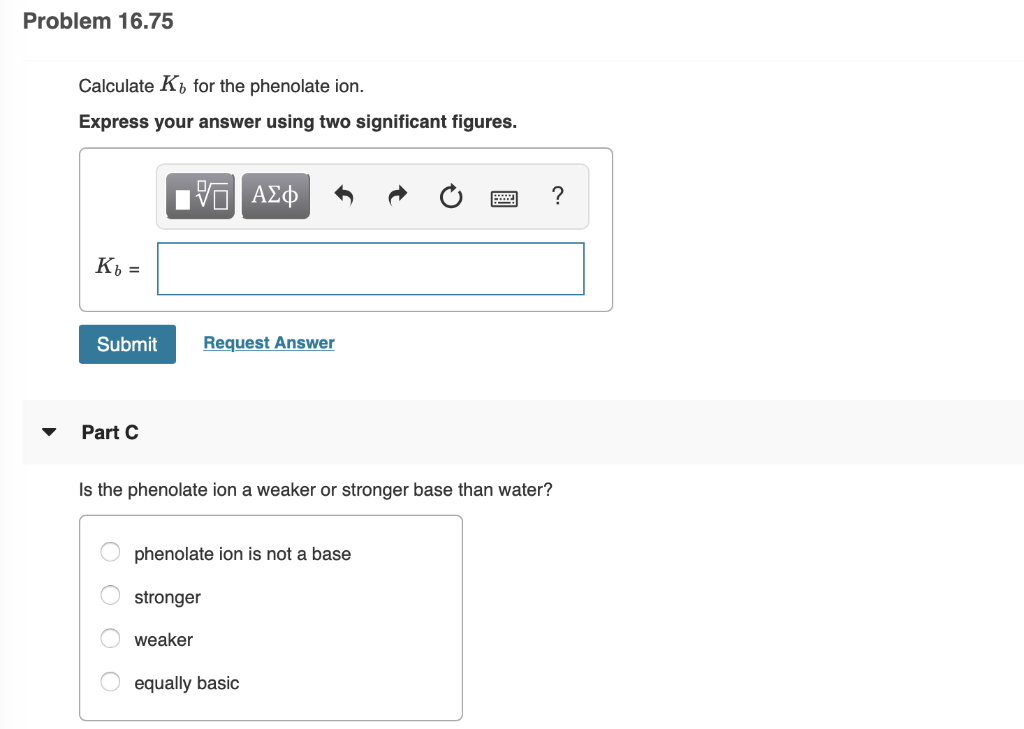
. First week only 499. B Calculate Kb for phenols conjugate base. Calculate Kb for phenols conjugate base.
Phenol is a weak aromatic acid. B Calculate Kbfor phenols conjugate base. In part b students were asked to select pH values of a buffered solution that would ensure more than 50 of the phenol was in its deprotonated form C 6 H 5 O aq.
1Write out the Ka reaction for phenol. Calculate Kb for phenols conjugate base. See the answer Phenol C6H5OH has a Ka of 13 10-10.
Phenol C6H5OH has a Ka of 13 10-10. The commercial product is a liquid. 1 For every A- there is one H3O.
Phenol C6H5OH has a Ka of 13 x10-10a Write out the Ka reaction for phenolb Calculate Kb for phenols conjugate basec Is phenol a stronger or weaker acid than water. Phenol C6H5OH partially dissociates in water as shown C6H5OH H2O -- H3O C6H5O- If Ka Questions Chem HELP ASAP Phenol C6H5OH partially dissociates in water as shown C6H5OH H2O -- H3O C6H5O- If Ka is 1610-10 and the concentration of H3O and C6H5O are both 1010-5 M at equilibrium what would be the concentration of phenol. Ka H C 6 H 5 O - C 6 H 5 OH 2.
105 rows Ka and pKa Values of Acids Phenols Alcohols Amines. Express your answer as a chemical equation including phases. In order to get a reasonably quick reaction with benzoyl chloride the phenol is first converted into sodium phenoxide by dissolving it in sodium hydroxide solution.
It is a colorless-to-white solid when pure. 3Is phenol a stronger or weaker acid than water. Kb10 -14 Ka10 -14 1310 -10 7710 -5 3.
Solution for Phenol has a pka of 992 and a ρ value of 225. 1 Please move if I posted this in the wrong place A solution of phenol in water has a concentration of 47 g dm-3 C5H5OH aq -- H aq C5H5O- aq sorry about the arrows C6H5OH aq OH- aq -- C5H5O- aq H20 l Ka 13 x 10-10 mol dm-3 sorry Write an expression for the acid dissociation Ka of phenol. Write the equation for the reaction that goes with this equilibrium constant.
Answer 0 joetheelite Answer. Ka H3O2 HA whereby Ka 13 x 10-10 M given by question itself. Start your trial now.
The phenoxide ion reacts more rapidly with benzoyl chloride than the original phenol does but even so you have to shake it with benzoyl chloride for about 15 minutes. The value of Ka for phenol a weak acid C6H5OH is 10010-10. Calculate the pH of a 0393 M aqueous solution of phenol a weak acid C6H5OH Ka 1010-10 and the equilibrium concentrations of the weak acid and its conjugate base.
Ka is given as 1310-10. For example the bromination of phenol leads to 246-tribromophenol even in the absence of a Lewis acid Fig. 7 When discussing the GattermannKoch reaction on phenol substrates it has been said that O -acylation is a problematic competing reaction as in a phenol theres high electron density on the O atom However there are also numerous other reactions where phenol reacts with electrophiles on the benzene ring.
Part A Write out the Ka reaction for phenol. Weve got the study and writing resources you need for your assignments. Expert Answer 100 1 rating.
Phenol has a distinct odor that is sickeningly sweet and tarry. The activating power of the phenolic group can be decreased by converting the phenol to an ester which can be removed by hydrolysis once the electrophilic sub-stitution reaction has been carried out Fig. Part A Write out the Ka reaction for phenol.
This problem has been solved. A Write out the Ka reaction for phenol. Since the ester is a.
A Write out the Ka reaction for phenol. Phenol C6H5OH has a Ka of 13 10-10. Add your answer and earn points.
Express your answer as a chemical equation including phases. 1 See answer Add answer 5 pts Advertisement macohen8307 is waiting for your help. What is the value of Kb for its conjugate base C6H50.
CliffsNotes study guides are written by real teachers and professors so no matter what youre studying CliffsNotes can ease your homework headaches and help you score high on exams. A Write out the Ka reaction for phenol. 3Is phenol a stronger or weaker acid than water.
Solutions of phenol C 6 H 5 OH aq. In part a students were to calculate the pH of a 075 M solution of phenol given the K a value for this weak acid. A Write out the Ka reaction for phenol.
Therefore A- is always equal H3O 2 H2O is constant ie. First week only 499. This problem has been solved.
C Is phenol a stronger or weaker acid than water. Question thumb_up100 Phenol C6H5OH has a Kaof 13 x10-10. 1Write out the Ka reaction for phenol.
Ka H3O A- HA H2O Key points. Phenol is both a manufactured chemical and a natural substance. You can taste and smell phenol at levels lower than those that are associated with harmful effects.
C 6 H 5 OH H 2 O --- C 6 H 5 O - H 3 O bK b C6H5OK w K a C. H2O 1 because water is hardly altered by the ionisation of HA Therefore the expression for the Ka of phenol is.

Dft Study Of Phenol Alkylation With Propylene On H Bea In The Absence And Presence Of Water Reaction Chemistry Engineering Rsc Publishing Doi 10 1039 D1re00201e
Solved Phenol C6h5oh Has A Ka Of 1 3 X 10 10 Part A Chegg Com
Solved Phenol C6h5oh Has A Ka Of 1 3 X 10 10 Part A Chegg Com

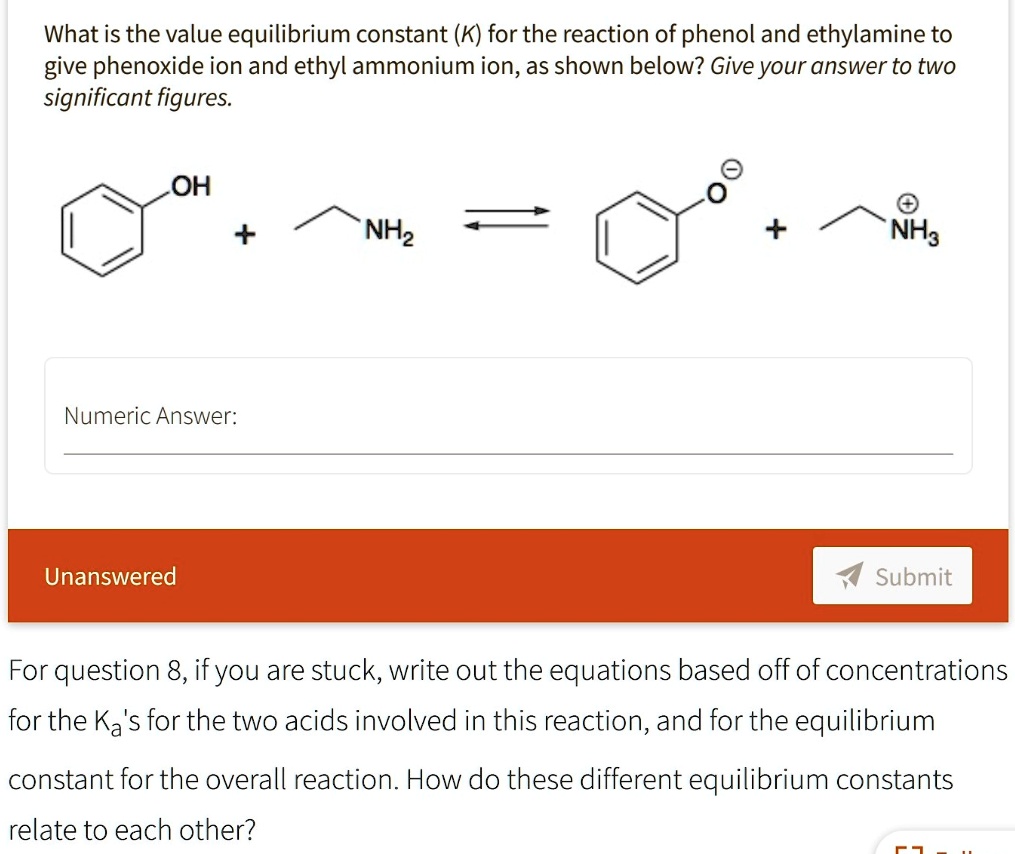
Solved What Is The Value Equilibrium Constant K For The Reaction Of Phenol And Ethylamine To Give Phenoxide Ion And Ethyl Ammonium Ion As Shown Below Give Your Answer To Two Significant Figures
No comments for "Write Out the Ka Reaction for Phenol."
Post a Comment